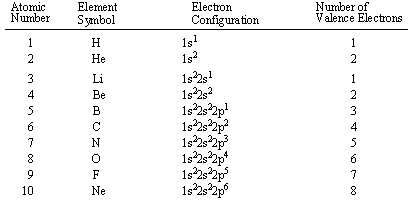
How many electrons fit in each shell around an atom? C4 transmission.
- Number Of Electrons 1s
- Number Of Electrons In Boron
- Number Of Electrons In Carbon
- Number Of Electrons In Lithium
The maximum number of electrons that can occupy a specific energy level can be found using the following formula:
Number Of Electrons 1s
This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell. Thus the s subshell has only one orbital, the p subshell has three orbitals, and so on. Spin Quantum Number (m s): m s = +½ or -½. Specifies the orientation of the spin axis of an electron. Therefore, a maximum number of #10# electrons can share these two quantum numbers in an atom. #n=3, l=2# These electrons are located on the third energy level, in the #3d# subshell.
Electron Capacity = 2n2
The variable n represents the Principal Quantum Number, the number of the energy level in question.
| Energy Level (Principal Quantum Number) | Shell Letter | Electron Capacity |
| 1 | K | 2 |
| 2 | L | 8 |
| 3 | M | 18 |
| 4 | N | 32 |
| 5 | O | 50 |
| 6 | P | 72 |
Keep in mind that an energy level need not be completely filled before electrons begin to fill the next level. You should always use the Periodic Table of Elements to check an element's electron configuration table if you need to know exactly how many electrons are in each level.
Number Of Electrons In Boron
Related Pages:
For questions about this page, please contact Steve Gagnon.
Latest version: 6.8.0, released on. Best for: Removing DRM from Kindle. Calibre: The one stop solution for all your e-book needs. Comprehensive e-book software. Part 1: Calibre DeDRM Plugin Will Remove DRM From? Kindle ebooks (Files from Kindle for. If you are using macOS older than 10.14 (Mojave), the last version of calibre that will work on your machine is 3.48, available here. If you are using macOS 10.8 (Mountain Lion), the last version of calibre that will work on your machine is 2.85.1, available here. Calibre osx.
How to calculate the number of electrons in an ion
To calculate the number of electrons in an ion that carries a positive charge, you must recall that when an ion carries a positive charge, the number of electrons are fewer than the number of protons. So to get the number of electrons, you must subtract the size of charge (which is often written as a superscript on the right side of the symbol) from the atomic number or proton number. Let's use figure 2 as an example. As you can see, Mg has an atomic number of 12 and a charge of +2, so to get the number of electrons for Mg ion, we must subtract 2 from the atomic number. Thus, 12-2= 10 electrons.
Number Of Electrons In Carbon
Likewise, to calculate the number of electrons for an ion that carries a negative charge, you must recall that when an ion carries a negative charge, the number of electrons are more than the number of protons. So to get the number of electrons, you must add the size of charge to the atomic or proton number. So from figure 3, the number of electrons for chloride ion is 17 + 1 = 18 electrons.
To learn how atoms form ions, click here.
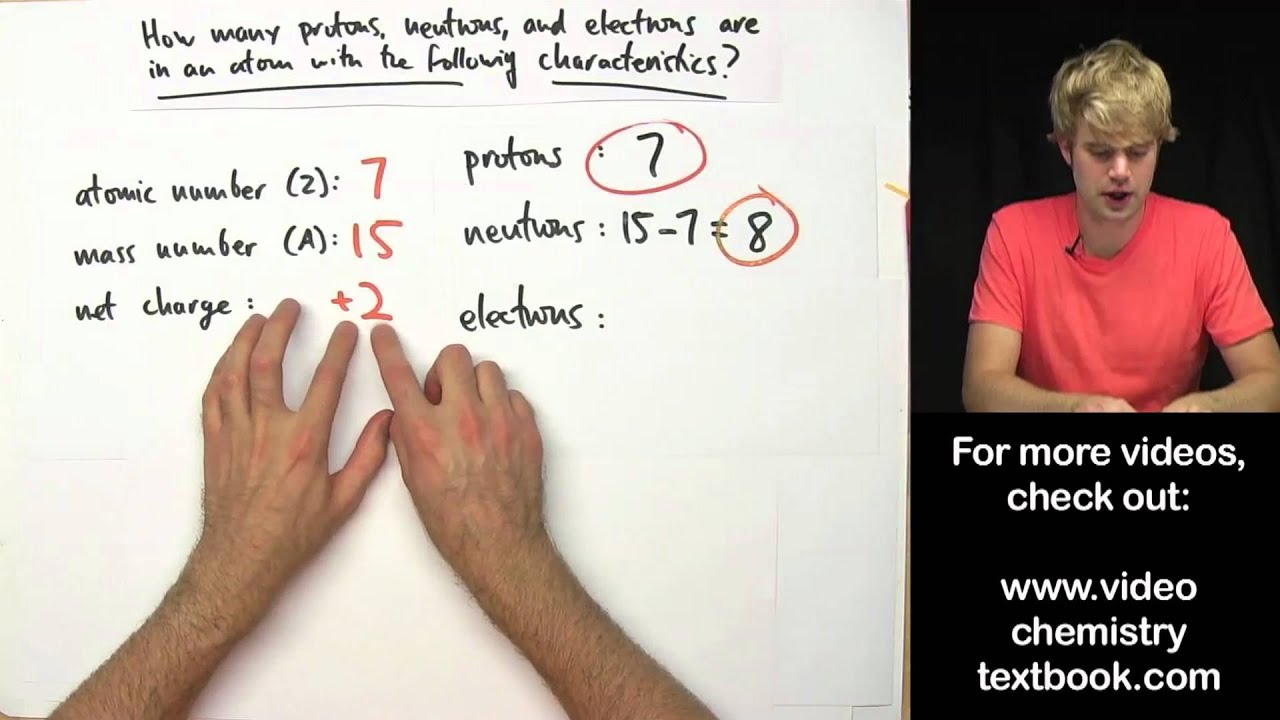
To calculate the number of electrons in an ion that carries a positive charge, you must recall that when an ion carries a positive charge, the number of electrons are fewer than the number of protons. So to get the number of electrons, you must subtract the size of charge (which is often written as a superscript on the right side of the symbol) from the atomic number or proton number. Let's use figure 2 as an example. As you can see, Mg has an atomic number of 12 and a charge of +2, so to get the number of electrons for Mg ion, we must subtract 2 from the atomic number. Thus, 12-2= 10 electrons.
Number Of Electrons In Carbon
Likewise, to calculate the number of electrons for an ion that carries a negative charge, you must recall that when an ion carries a negative charge, the number of electrons are more than the number of protons. So to get the number of electrons, you must add the size of charge to the atomic or proton number. So from figure 3, the number of electrons for chloride ion is 17 + 1 = 18 electrons.
To learn how atoms form ions, click here.
To learn more about subatomic particles, click here
Number Of Electrons In Lithium
To learn why a cation is smaller than its neutral atom, click here Knives out 2019 full movie watch online.

