
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. More than 99.94% of an atom's mass is in the nucleus. Atoms Versus Molecules. When atoms bond together, they become molecules. When the chemical symbol of a molecule is written out, you can distinguish it from an atom by the subscript following the element symbol, which indicates how many atoms are present. All atoms are: a. Positively charged, with the number of protons exceeding the number of electrons. Negatively charged, with the number of electrons exceeding the number of protons.
- Identify the main points of Dalton's atomic theory
Key Points
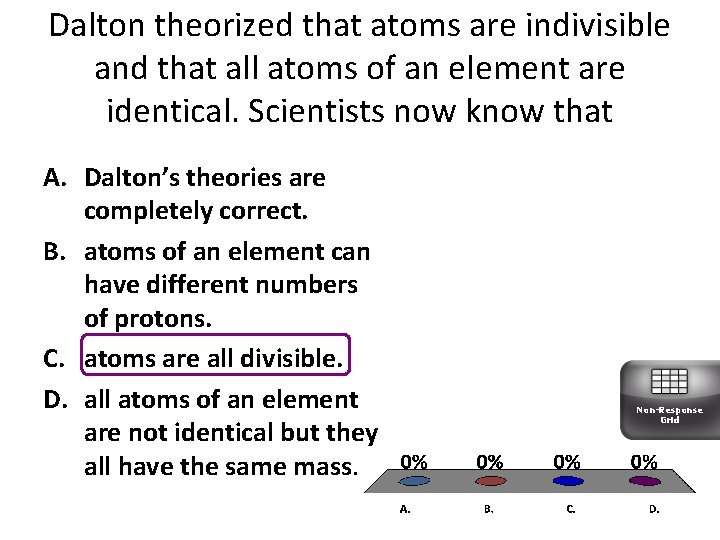
- Dalton's atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass.
- Dalton's atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios.
- Dalton also postulated that chemical reactions resulted in the rearrangement of the reacting atoms.
Terms
- atomic mass unitThe standard unit that is used for indicating mass of an atom.
- atomThe smallest possible amount of matter that still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.
History of Dalton's Atomic Theory
Although the concept of the atom dates back to the ideas of Democritus, the English meteorologist and chemist John Dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of Antoine Lavoisier and Joseph Proust.
Proust had studied tin oxides and found that their masses were either 88.1% tin and 11.9% oxygen or 78.7% tin and 21.3% oxygen (these were tin(II) oxide and tin dioxide respectively). Dalton noted from these percentages that 100g of tin will combine either with 13.5g or 27g of oxygen; 13.5 and 27 form a ratio of 1:2. Dalton found an atomic theory of matter could elegantly explain this common pattern in chemistry – in the case of Proust's tin oxides, one tin atom will combine with either one or two oxygen atoms.
Dalton also believed atomic theory could explain why water absorbed different gases in different proportions: for example, he found that water absorbed carbon dioxide far better than it absorbed nitrogen. Dalton hypothesized this was due to the differences in the mass and complexity of the gases' respective particles. Indeed, carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). Since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.
Dalton's Atomic Theory
The main points of Dalton's atomic theory are: Atomic no.

- Everything is composed of atoms, which are the indivisible building blocks of matter and cannot be destroyed.
- All atoms of an element are identical.
- The atoms of different elements vary in size and mass.
- Compounds are produced through different whole-number combinations of atoms.
- A chemical reaction results in the rearrangement of atoms in the reactant and product compounds.
All Atoms Are In Constant Motion
Atomic theory has been revised over the years to incorporate the existence of atomic isotopes and the interconversion of mass and energy. In addition, the discovery of subatomic particles has shown that atoms can be divided into smaller parts. However, Dalton's importance in the development of modern atomic theory has been recognized by the designation of the atomic mass unit as a Dalton.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://www.boundless.com//chemistry/definition/atomic-mass-unit
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/atom
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Atomic_theory
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/John_Dalton
Wikipedia
CC BY-SA 3.0.
Many people might think atoms and elements are the same. However, atoms and elements do have a few differences when you start breaking it down. The main difference is elements are made of atoms. Learn other differences between atoms and elements by dissecting these two terms. Explore examples of elements and atoms.
What Is an Atom?
You might have heard the term atom thrown around in chemistry. And, there's a good reason for that; it's extremely important. Every compound, molecule, or element you come across will be made of atoms. For example, humans are made of atoms. Air is made of atoms. Your computer… made of atoms. Everything is made of atoms.
This is why you can't begin to understand the difference between atoms and elements without first understanding what an atom is and what it's made of. Simply put, atoms are the building blocks of elements. They are some of the smallest bits of what you would call ordinary matter.

Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. More than 99.94% of an atom's mass is in the nucleus. Atoms Versus Molecules. When atoms bond together, they become molecules. When the chemical symbol of a molecule is written out, you can distinguish it from an atom by the subscript following the element symbol, which indicates how many atoms are present. All atoms are: a. Positively charged, with the number of protons exceeding the number of electrons. Negatively charged, with the number of electrons exceeding the number of protons.
- Identify the main points of Dalton's atomic theory
Key Points
- Dalton's atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass.
- Dalton's atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios.
- Dalton also postulated that chemical reactions resulted in the rearrangement of the reacting atoms.
Terms
- atomic mass unitThe standard unit that is used for indicating mass of an atom.
- atomThe smallest possible amount of matter that still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.
History of Dalton's Atomic Theory
Although the concept of the atom dates back to the ideas of Democritus, the English meteorologist and chemist John Dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of Antoine Lavoisier and Joseph Proust.
Proust had studied tin oxides and found that their masses were either 88.1% tin and 11.9% oxygen or 78.7% tin and 21.3% oxygen (these were tin(II) oxide and tin dioxide respectively). Dalton noted from these percentages that 100g of tin will combine either with 13.5g or 27g of oxygen; 13.5 and 27 form a ratio of 1:2. Dalton found an atomic theory of matter could elegantly explain this common pattern in chemistry – in the case of Proust's tin oxides, one tin atom will combine with either one or two oxygen atoms.
Dalton also believed atomic theory could explain why water absorbed different gases in different proportions: for example, he found that water absorbed carbon dioxide far better than it absorbed nitrogen. Dalton hypothesized this was due to the differences in the mass and complexity of the gases' respective particles. Indeed, carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). Since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.
Dalton's Atomic Theory
The main points of Dalton's atomic theory are: Atomic no.
Roku provides the simplest way to stream entertainment to your TV. With thousands of available channels to choose from. Roku streaming players start at just £29.99, and there are no monthly equipment fees or for watching free channels. You only pay for subscription channels like Netflix or movie and TV show rentals from services like Sky Store. Hi, I'm looking to make a purchase for a tv for my enclosed porch. The TV will be placed up in a corner and I don't want to turn it super-loud and annoy my neighbors (I'm hard of hearing) so I'm looking at options for speakers to be nearer to where we'll be sitting (approx. 15 ft away from the TV). Open the Sky Go app on your laptop Find what you want to watch and go fullscreen Attach the HDMI cable to your laptop Plug the other end into a free HDMI port on the back of the TV. Is it possible to get Sky Sports or Sky Go on Roku in the UK? I currently use Sky Go on my PS4, but am about to get rid of the PS4 and so trying to work out how I can continue to get Sky Go (I use my parent's login, don't have my own Sky).
- Everything is composed of atoms, which are the indivisible building blocks of matter and cannot be destroyed.
- All atoms of an element are identical.
- The atoms of different elements vary in size and mass.
- Compounds are produced through different whole-number combinations of atoms.
- A chemical reaction results in the rearrangement of atoms in the reactant and product compounds.
All Atoms Are In Constant Motion
Atomic theory has been revised over the years to incorporate the existence of atomic isotopes and the interconversion of mass and energy. In addition, the discovery of subatomic particles has shown that atoms can be divided into smaller parts. However, Dalton's importance in the development of modern atomic theory has been recognized by the designation of the atomic mass unit as a Dalton.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://www.boundless.com//chemistry/definition/atomic-mass-unit
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/atom
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Atomic_theory
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/John_Dalton
Wikipedia
CC BY-SA 3.0.
Many people might think atoms and elements are the same. However, atoms and elements do have a few differences when you start breaking it down. The main difference is elements are made of atoms. Learn other differences between atoms and elements by dissecting these two terms. Explore examples of elements and atoms.
What Is an Atom?
You might have heard the term atom thrown around in chemistry. And, there's a good reason for that; it's extremely important. Every compound, molecule, or element you come across will be made of atoms. For example, humans are made of atoms. Air is made of atoms. Your computer… made of atoms. Everything is made of atoms.
This is why you can't begin to understand the difference between atoms and elements without first understanding what an atom is and what it's made of. Simply put, atoms are the building blocks of elements. They are some of the smallest bits of what you would call ordinary matter.
Structure of an Atom
Like everything else, atoms have a few different things floating around inside of them. These subatomic particles include:
- neutrons - with no charge
- protons - positively charged
- electrons - negatively charged
The protons and neutrons are in the atomic nucleus of the atom, while the electrons orbit the atomic nucleus. Think of this as similar to the way the planets all orbit the sun. That's an atom in a nutshell. Well, if a nutshell was orbited by lots of little electrons, that is.
What Is an Element?
With atoms out of the way, it's time to look at elements. If you've ever seen a Periodic Table of Elements, then you probably have some idea of what elements are. But to break it down into a simple definition, elements are all the different types of atoms we know exist on Earth. They are arranged by their atomic number on the Periodic Table of Elements.
For example, gold is an element. If you were to hold a chunk of pure gold in your hand, you would be holding an element. Other elements include:
- Hydrogen
- Boron
- Carbon
- Neon
- Magnesium
- Silicon
- Aluminum
- Chloride
- Oxygen
- Calcium
All Atoms Are Made Of Three Types Of Particles
Elements and Atomic Number
What makes something an element is the fact that all the atoms have the same number of protons in the nucleus. While you can find them all on the periodic table, let's look at the common elements' mercury and copper.
- Mercury is an element with 80 protons in its nucleus. It has an atomic number of 80.
- Copper is made of atoms with 29 protons in the nucleus. Therefore, it has an atomic number of 29.
What Is the Difference Between an Element and a Molecule?
With atoms and elements all cleared up, it's important to understand the difference between a molecule and an element. Because like all things in the world, elements and molecules are both made of atoms. You know elements are all the different types of atoms on the periodic table. Molecules are what you get when those atoms are combined. Unlike elements, molecules can be made from the same or different elements. The key to a molecule is that two or more atoms are bonded together.
For example, water is a molecule made of hydrogen and oxygen. It's actually made of two hydrogen atoms and one oxygen atom. You can also have molecules of a single atom bonded together like two oxygen atoms. This makes up the oxygen humans breathe.
Difference Between Atoms and Elements
It can be easy to see why elements and atoms get confused because elements are atoms. They are just a group of all the same kind of atoms. Calibre osx. All the known elements on Earth can be found in the periodic table of elements.
Science is fun right? Keep the chemistry fun going by exploring the difference between atoms and molecules. You can also have more chemistry fun by reading up on molecules and compounds.
All Atoms Are Neutral
Certified Teacher

